Benzene is aromatic due to its unique structure and the delocalization of its six π electrons. According to Hückel's Rule, a compound is aromatic if it contains (4n + 2) π electrons, and benzene fits this criterion perfectly. Its cyclic, planar nature and full conjugation contribute to its stability, making it more stable than typical unsaturated compounds. The resonance within benzene equalizes bond lengths, enhancing this stability. This unique electronic arrangement also affects its reactivity, allowing for specific substitution reactions. There's a lot more to discover about benzene and its fascinating properties.
Key Takeaways
- Benzene is cyclic and planar, allowing for effective overlap of p-orbitals and delocalization of π electrons.
- It contains six π electrons, satisfying Hückel's Rule of (4n + 2) for aromatic compounds.
- The resonance in benzene results in equal carbon-carbon bond lengths, enhancing stability and confirming its aromatic classification.
- Aromatic compounds like benzene are more stable than expected due to their delocalized electron system, leading to lower heat of hydrogenation.
- Benzene primarily undergoes electrophilic substitution reactions, which preserve its aromatic stability.
Definition of Aromaticity

Aromaticity is a key concept in organic chemistry that describes the unique stability of certain cyclic compounds.
You'll find that aromatic compounds, like benzene, have a ring structure that allows for the delocalization of π electrons across a continuous loop of p-orbitals. This delocalization is essential for achieving aromatic stability.
According to Hückel's Rule, a compound is considered aromatic if it contains (4n + 2) π electrons, where n is a non-negative integer. Benzene, with six π electrons, fits this rule perfectly.
The resonance in benzene results in equal bond lengths, enhancing its overall stability. This unique arrangement not only defines its aromatic nature but also contributes to its distinctive chemical properties.
Hückel's Rule Explained
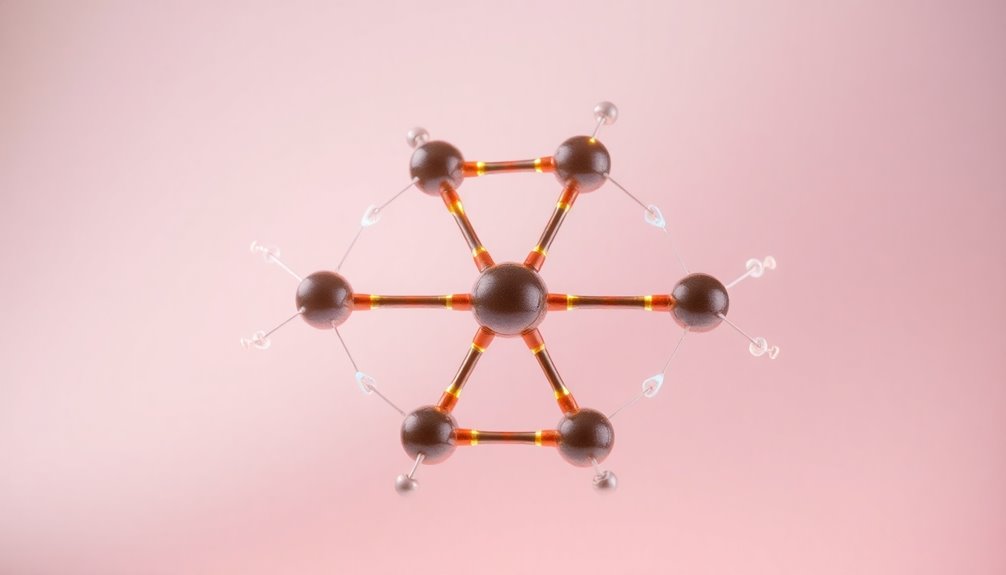
Understanding the stability of benzene and other aromatic compounds hinges on Hückel's Rule. This rule states that a compound is aromatic if it's cyclic, planar, fully conjugated, and contains (4n + 2) π electrons, where n is a non-negative integer.
For benzene, with its 6 π electrons, n equals 1, confirming its aromatic nature. The delocalized π electrons across the benzene ring contribute to its exceptional resonance stabilization, resulting in equal bond lengths that sit between single and double bonds.
This stability distinguishes aromatic compounds from antiaromatic compounds, which have 4n π electrons and are significantly less stable. Hückel's Rule is essential in understanding why benzene remains a cornerstone of aromatic chemistry.
Structure of Benzene
Benzene's structure features a unique hexagonal ring made up of six carbon atoms, each bonded to a hydrogen atom.
This arrangement allows for a delocalized electron system that enhances the molecule's stability and aromatic properties.
You'll find that the uniform bond lengths reflect the resonance characteristics that define benzene's true nature.
Hexagonal Ring Structure
While exploring the structure of benzene, you'll discover its distinctive hexagonal ring, which consists of six carbon atoms arranged in a flat configuration. Each carbon atom is sp² hybridized, promoting effective overlap of p-orbitals.
You'll notice that every carbon-carbon bond measures 1.40 Å, reflecting resonance and the delocalization of electrons throughout the ring. This equal distribution of π electrons contributes greatly to benzene's stability.
The cyclic and planar nature of the hexagonal structure allows benzene to satisfy Hückel's rule, further affirming its classification as an aromatic compound.
This unique hexagonal ring structure is key to understanding why benzene exhibits such remarkable stability and aromatic properties, setting it apart from other hydrocarbons.
Delocalized Electron System
The hexagonal ring structure of benzene sets the stage for its unique delocalized electron system, which plays a vital role in its aromatic properties. Each carbon atom contributes to a network of bonds, allowing for the delocalization of six π electrons. This electron delocalization adheres to Hückel's rule, ensuring stability with the formula 4n + 2 (n=1). The planar geometry of benzene facilitates effective overlap of p-orbitals, enhancing resonance stabilization. As a result, all carbon-carbon bonds are equal in length (1.40 Å), reflecting a uniform bond character.
| Feature | Description | Importance |
|---|---|---|
| Delocalized Electrons | π electrons spread across the ring | Contributes to aromaticity |
| Resonance | Multiple structures exist | Enhances stability |
| Geometry | Planar hexagonal shape | Allows effective overlap |
Properties of Benzene

When you explore the properties of benzene, you'll notice its unique stability mechanism that sets it apart from non-aromatic compounds.
Its ability to undergo electrophilic substitution reactions helps maintain this stability while acting as a non-polar solvent.
This distinct combination of characteristics makes benzene a fascinating compound in the world of chemistry.
Unique Stability Mechanism
Benzene's unique stability stems from the delocalization of its six π electrons throughout its cyclic structure, which creates a phenomenon known as resonance. This delocalization leads to bond lengths of 1.40 Å, indicating a hybrid character of the carbon-carbon bonds. According to Hückel's Rule, benzene, with its 4n + 2 π electrons (n = 1), is classified as aromatic. Its stability is remarkable, being 36 kcal/mole more stable than expected from hydrogenation measurements of related alkenes. This exceptional stability allows benzene to retain its aromatic nature by favoring electrophilic substitution reactions.
| Property | Description | Significance |
|---|---|---|
| π Electrons | 6 π electrons | Delocalization enhances stability |
| Bond Lengths | All equal at 1.40 Å | Indicates hybrid character |
| Aromatic Classification | Fulfills Hückel's Rule | Confirms unique stability |
Electrophilic Substitution Reactions
Electrophilic substitution reactions allow benzene to maintain its aromatic stability while engaging with various electrophiles. Instead of addition reactions, benzene undergoes these substitutions because its stable aromatic structure preserves its aromaticity.
The high electron density of the benzene ring attracts electrophiles, which attack the π electrons. Common examples include nitration, producing nitrobenzene, and sulfonation, yielding benzenesulfonic acid. Catalysts like sulfuric acid are often necessary to facilitate these reactions by generating the electrophile.
Benzene's unique resonance stabilization enables substitution at different positions—ortho, meta, or para—depending on existing substituents, influencing product distribution. This balance of reactivity and stability highlights benzene's remarkable ability to engage in electrophilic reactions while retaining its aromatic characteristics.
Non-Polar Solvent Properties
Aromatic compounds like benzene serve as excellent non-polar solvents due to their symmetrical structure and lack of significant dipole moments. This non-polarity makes benzene effective for dissolving non-polar or weakly polar compounds.
- It has a low boiling point (80.1 °C), making it ideal for various chemical reactions.
- Benzene's low viscosity allows for easy handling, enhancing its utility in applications.
- It's widely used in extraction and chromatography processes to dissolve a range of organic compounds.
Because benzene doesn't mix with polar solvents like water, it effectively extracts non-polar substances.
Its unique properties make it a valuable choice in laboratories and industries alike, showcasing the versatility of this aromatic solvent.
Reactivity of Aromatic Compounds

When studying the reactivity of aromatic compounds, it's vital to understand that they primarily undergo electrophilic substitution reactions instead of addition reactions. This allows benzene and other aromatic compounds to retain their aromaticity during chemical transformations.
The delocalized π electrons contribute to their enhanced stability, making them less reactive than alkenes. Substituents play an important role; electron-donating groups increase electron density, activating the ring toward electrophilic attack, while electron-withdrawing groups decrease reactivity.
The unique stability of aromatic compounds is reflected in their lower heat of hydrogenation compared to non-aromatic compounds, indicating they're more stable than saturated counterparts. This stability enables a wide range of substitution reactions, producing diverse derivatives that maintain the aromatic character.
Common Benzene Derivatives

Benzene derivatives are essential in various industries due to their unique chemical properties and applications. These substituted compounds contribute greatly to manufacturing and chemical processes.
- Toluene: A methylbenzene where one hydrogen atom is replaced by a methyl group, enhancing its solvent properties.
- Xylene: With two methyl groups on the benzene ring, it comes in three isomers (ortho, meta, and para), each exhibiting distinct properties.
- Styrene: This derivative serves as a precursor for polystyrene, a widely used polymer.
Other notable derivatives include chlorobenzene, used as a solvent, and nitrobenzene, important in synthesizing dyes and pharmaceuticals.
The diverse applications of these aromatic derivatives highlight their importance in both industrial and commercial contexts.
Nomenclature of Aromatic Compounds
Understanding the significance of benzene derivatives sets the stage for exploring how these compounds are named. The nomenclature of aromatic compounds follows IUPAC guidelines, where "benzene" serves as the base name for compounds with a benzene ring.
For monosubstituted aromatic compounds, you name the substituent before "benzene," like toluene for methylbenzene. In disubstituted compounds, you indicate the positions of substituents using ortho (1,2-), meta (1,3-), and para (1,4-).
Additionally, the "phenyl" prefix comes into play when a benzene ring attaches to a longer carbon chain, such as in phenylethylamine. Certain functional groups, like chlorine, amino, and nitro, have specific naming conventions, leading to terms like chlorobenzene and nitrobenzene.
Applications in Industry
Aromatic compounds, particularly benzene and its derivatives, play an essential role in various industrial applications.
You'll find benzene as a key precursor in the synthesis of many industrial chemicals, especially styrene, which is important for creating polystyrene, a widely used plastic. Its non-polar nature makes benzene an effective solvent in diverse industrial processes.
- Benzene derivatives like toluene and xylene serve as solvents and paint thinners.
- The aromatic structure of benzene is critical in pharmaceuticals, acting as a building block for many active pharmaceutical ingredients (APIs).
- Benzene and its derivatives are also significant in the production of synthetic fibers, including nylon and polyester, greatly impacting the textile industry. Additionally, the use of essential oils in various applications highlights their versatility in both therapeutic and industrial settings.
Environmental Considerations

While many industries rely on benzene and its derivatives, the environmental implications of these compounds can't be ignored.
Benzene, an aromatic compound, is a common pollutant found in industrial waste and vehicle emissions. Its stability makes it resistant to degradation, allowing it to persist in the environment. This leads to significant environmental impact, including the potential for benzene to bioaccumulate in aquatic organisms, disrupting ecosystems and food chains.
As a classified carcinogen, benzene raises health concerns, prompting strict regulations to limit its release. Groundwater contamination poses long-term challenges, complicating remediation efforts and increasing costs.
It's essential to remain aware of these factors to protect both public health and the environment from benzene's harmful effects.
Frequently Asked Questions
Why Is Benzene an Aromatic Molecule?
You're asking why benzene's special.
This molecule has a unique structure; its six carbon atoms form a flat, cyclic arrangement. Each atom shares electrons, creating a system of delocalized π electrons.
You'll notice that all the carbon-carbon bonds have equal lengths, which suggests stability. Plus, benzene's lower heat of hydrogenation shows it's more stable than expected.
This unique combination of features is what makes benzene stand out as an aromatic compound.
Why Is Benzyne Aromatic?
You'll find that benzyne is aromatic due to its planar, cyclic structure, which allows for the delocalization of π electrons.
With six π electrons, it meets Hückel's rule, confirming its aromatic stability.
The resonance structures reveal how electron density spreads throughout the ring, enhancing stability.
Even with a triple bond, the overall electron delocalization remains intact, enabling benzyne to undergo electrophilic substitution reactions typical of aromatic compounds.
How Will You Confirm That Benzene Is Aromatic?
To confirm that benzene is aromatic, you'll need to check its structure and electron count.
Look for a cyclic, planar arrangement of carbon atoms. Then, count the π electrons; you should find six, which fits Hückel's rule (4n + 2).
Additionally, observe the uniform bond lengths of 1.40 Å, indicating resonance and electron delocalization.
Finally, compare its hydrogenation energy to typical alkenes to see its increased stability.
Why Is Benzene an Aromatic Compound but Cyclohexane Is Not?
Imagine you're at a retro diner, sipping a milkshake while pondering why benzene's got that aromatic flair and cyclohexane doesn't.
Benzene's a cyclic compound with delocalized π electrons, allowing for resonance stabilization and equal bond lengths. In contrast, cyclohexane's all about single bonds and lacks π electrons, making it non-aromatic and less stable.
The planar structure of benzene fosters effective overlap of p-orbitals, which cyclohexane's chair conformation just can't achieve.
Conclusion
To sum up, benzene's aromatic nature isn't just a textbook concept; it's a real-life phenomenon that shapes everything from fragrances to pharmaceuticals. You might be surprised to learn that despite its sweet scent, benzene can be harmful. So, while you enjoy that delightful aroma or marvel at its intricate structure, remember that understanding its properties and reactivity helps us appreciate both its beauty and its risks in our daily lives. Aromatic compounds are indeed fascinating!